|
||
| Sulphuric Acid on the WebTM | Technical Manual | DKL Engineering, Inc. |
Knowledge for the
Sulphuric Acid Industry
![]()
Sulphuric Acid on the Web
Introduction
General
Equipment Suppliers
Contractor
Instrumentation
Industry News
Maintenance
Acid
Traders
Organizations
Fabricators
Conferences
Used
Plants
Intellectual
Propoerty
Acid
Plant Database
Market
Information
Library
Technical Manual
Introduction
General
Definitions
Instrumentation
Plant Safety
Metallurgial
Processes
Metallurgical
Sulphur Burning
Acid Regeneration
Lead Chamber
Technology
Gas Cleaning
Contact
Strong Acid
Acid Storage
Loading/Unloading
Transportation
Sulphur
Systems
Liquid SO2
Boiler Feed Water
Steam Systems
Cooling Water
Effluent Treatment
Utilities
Construction
Maintenance
Inspection
Analytical Procedures
Materials of Construction
Corrosion
Properties
Vendor Data
DKL Engineering, Inc.
Handbook of Sulphuric Acid Manufacturing
Order
Form
Preface
Contents
Feedback
Sulphuric Acid
Decolourization
Order Form
Preface
Table of Contents
Process Engineering Data Sheets - PEDS
Order
Form
Table of Contents
Introduction
Bibliography of Sulphuric Acid Technology
Order Form
Preface
Contents
Technology - CASOX
April 9, 2003
|
Introduction |
Associated Links |
The CASOX Process is a SOx
removal process using catalytic SOx-Oxidation using a newly developed high
performance de-sulphurizing catalyst. The CASOX Process leads to
significant reductions in construction and operating costs, compared with
conventional flue gas de-sulphurization processes such as lime scrubbing.
Concentrated sulphuric acid, gypsum and ammonium sulphate can be produced as
by-products of this process.
The process is easy to operate
and maintain since the gas to be treated is simply passed though the catalyst
bed. The use of a monolith catalyst (i.e. honeycomb) enables the process
to handle gases containing dust.
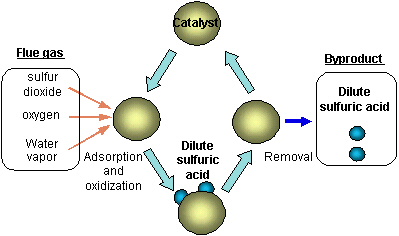
The CASOX Process adsorbs sulphur
dioxide gas and oxidizes it with oxygen to form sulphur trioxide. The
presence of water vapour results in the formation of sulphuric acid which is
removed and recovered. The concentration of sulphuric acid is
approximately 60%. Since the acid formed is continuously removed, there is
no requirement to regenerate the catalyst.